Enantiomeric excess of 1,2-diols by formation of cyclic boronates: an improved method |
| |
| Affiliation: | 1. Key Laboratory of Nondestructive Testing Ministry of Education, School of the Testing and Photoelectric Engineering, Nanchang Hangkong University, Nanchang 330063, China;2. Key Laboratory of Novel Thin-Film Solar Cells, Division of Solar Energy Materials and Engineering, Institute of Plasma Physics, Chinese Academy of Sciences, Hefei, Anhui 230031, China;3. State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources, North China Electric Power University, Beijing 102206, China;1. Nuclear Science & Technology Development Center, National Tsing Hua University, Hsinchu 30013, Taiwan;2. Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan;3. Department of Chemistry, National Central University, Chungli 32001, Taiwan;1. Department of Physiology, Medical College of Georgia at Augusta University, Augusta, GA 30912, United States;2. Department of Medicine, Renal Division, Emory University, 615 Michael St. Ste 605C, Atlanta, GA 30322, United States;3. Center for Cardiovascular Research, The Research Institute at Nationwide Children’s Hospital, Columbus, OH 43215, United States;4. Pharmacology Department, Medical School of Ribeirão Preto, University of São Paulo, Av Bandeirantes 3900, Ribeirão Preto, SP 14049-900, Brazil;1. School of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK;2. Department of Chemistry, College of Science, University of Sulaimani, Sulaimanyah, Kurdistan Region, Iraq;3. Komar Research Center (KRC), Komar University of Science and Technology, Sarchinar, Qularaisi District, Sulaimani, 46001, Kurdistan Region, Iraq;1. Division of Advanced Materials, Korea Research Institute of Chemical Technology, Daejeon 34114, Republic of Korea;2. Department of Polymer Science and Engineering, Chungnam National University, Daejeon 34134, Republic of Korea;3. Department of Chemical Engineering, Hankyong National University, Anseong 17579, Republic of Korea;1. Graduate School of Pharmaceutical Sciences, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba 260-8675, Japan;2. Molecular Chirality Research Center, Chiba University, 1-33, Yayoi-cho, Inage-ku, Chiba 263-8522, Japan |
| |
| Abstract: | 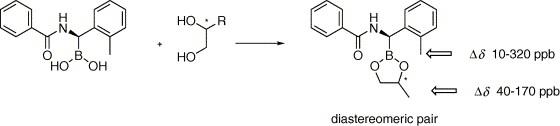
A reliable method for determining the enantiomeric composition of 1,2-diols by the formation of diastereomeric cyclic esters with boronic acid is described. Starting from a previously reported structure of boronic chiral derivatizing agent (CDA), seven structurally related racemic CDAs were synthesized and their discriminating ability towards diols measured. The most promising amongst these was synthesized in its enantiomerically pure form according to Matteson’s protocol for the stereoselective homologation of pinanediol boronates; this CDA quantitatively and rapidly reacts with 1,2-diols in very mild conditions affording a couple of diastereoisomers, whose composition can be determined via 1H NMR analysis. In particular, an attractive feature is that the resonance used for the analysis originated from the CDA as a couple of baseline-separated singlets (Δδ up to 0.3 ppm) is useful for integration. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

