Dimethylenebicyclo[1.1.1]pentanone |
| |
| Affiliation: | 1. Facultad de Química, Universidad Nacional Autónoma de México, Cd. Universitaria, Coyoacán, C.P. 04510 México D.F., Mexico;2. Departamento de Electroquímica, Centro de Investigación y Desarrollo Tecnológico en Electroquímica, S.C., Parque Tecnológico Querétaro, Sanfandila, Pedro de Escobedo C.P. 76703, Queretaro, Mexico;1. Institute of Problems of Chemical Physics, Russian Academy of Sciences, 142432 Chernogolovka, Moscow Region, Russian Federation;2. Lehrstuhl für Organische Chemie II, Ruhr-Universität, D-44780, Bochum, Germany;1. School of Mathematics and Computing Science, Changsha University of Science and Technology, Changsha, 410114, PR China;2. School of Mathematics and Statistics, Lanzhou University, Lanzhou 730000, PR China;1. Department of Chemistry, Faculty of Science, Banaras Hindu University, Varanasi 221005, India;2. Department of Chemistry, Loughborough University, Loughborough, Leics LE11 3TU, UK |
| |
| Abstract: | 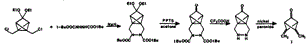
The highly strained and reactive dimethylenebicyclo[1.1.1]pentanone (I), was prepared as follows. Treatment of 1,5-bis(chloromethyl)tricyclo[2.1.0.02, 5]pentan-3-one diethyl ketal (III) with di-t-butyl hydrazodicarboxylate (VII), followed by pyridinium p-toluenesulfonate and trifluoroacetic acid, yielded the keto hydrazine X which was oxidized to the desired ketone I. The ketone I decomposes very rapidly above −30°C. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|