

高等学校化学学报 ›› 2019, Vol. 40 ›› Issue (5): 855.doi: 10.7503/cjcu20180688
收稿日期:2018-10-15
出版日期:2019-05-06
发布日期:2019-07-04
作者简介:联系人简介: 豆义波, 男, 博士, 副研究员, 主要从事金属有机框架基复合功能材料研究. E-mail:
基金资助:
HE Pengchen, ZHOU Jian, ZHOU Awu, DOU Yibo*( ), LI Jianrong*(
), LI Jianrong*( )
)
Received:2018-10-15
Online:2019-05-06
Published:2019-07-04
Contact:
DOU Yibo,LI Jianrong
E-mail:douyb@bjut.edu.cn;jrli@bjut.edu.cn
Supported by:摘要:
系统总结了金属有机框架(MOFs)基材料在光催化还原CO2中的最新研究进展, 其中包括MOFs直接作为光催化剂和作为复合光催化2个主要部分, 讨论了MOFs基光催化剂在催化还原CO2方面展现出的独特优势, 并对MOFs基光催化剂的结构稳定性与CO2转化效率等问题进行讨论与分析, 对未来发展趋势进行了展望.
中图分类号:
TrendMD:
何鹏琛, 周健, 周阿武, 豆义波, 李建荣. MOFs基材料在光催化CO2还原中的应用. 高等学校化学学报, 2019, 40(5): 855.
HE Pengchen,ZHOU Jian,ZHOU Awu,DOU Yibo,LI Jianrong. MOFs-Based Materials for Photocatalytic CO2 Reduction†. Chem. J. Chinese Universities, 2019, 40(5): 855.
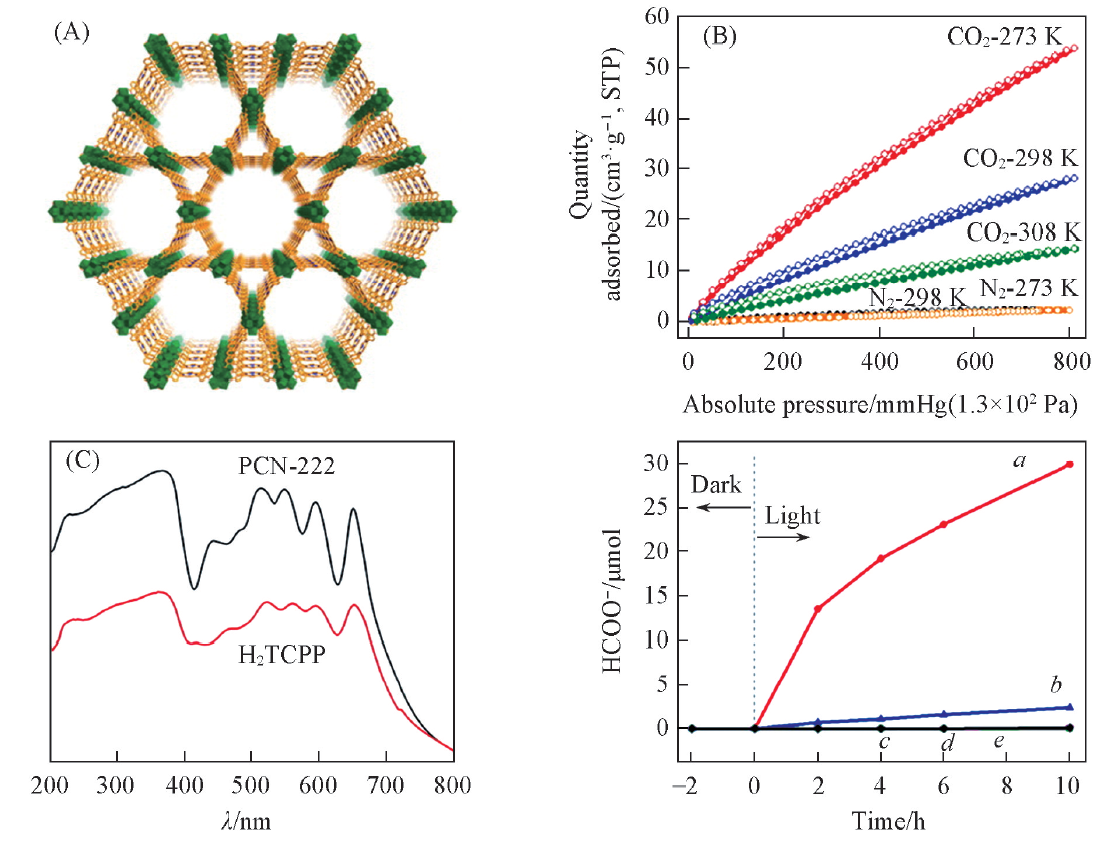
Fig.1 Structure(A) and CO2 and N2 adsorption isotherms(B) of PCN-222, UV-Vis spectra of PCN-22 and H2TCPP(C) and the amount of HCOO-(D) with PCN-22 as catalyst[33] (D) a. PCN-222; b H2TCPP; c. no PCN-222; d. no TEOA; e. no CO2. Copyright 2015, American Chemical Society.
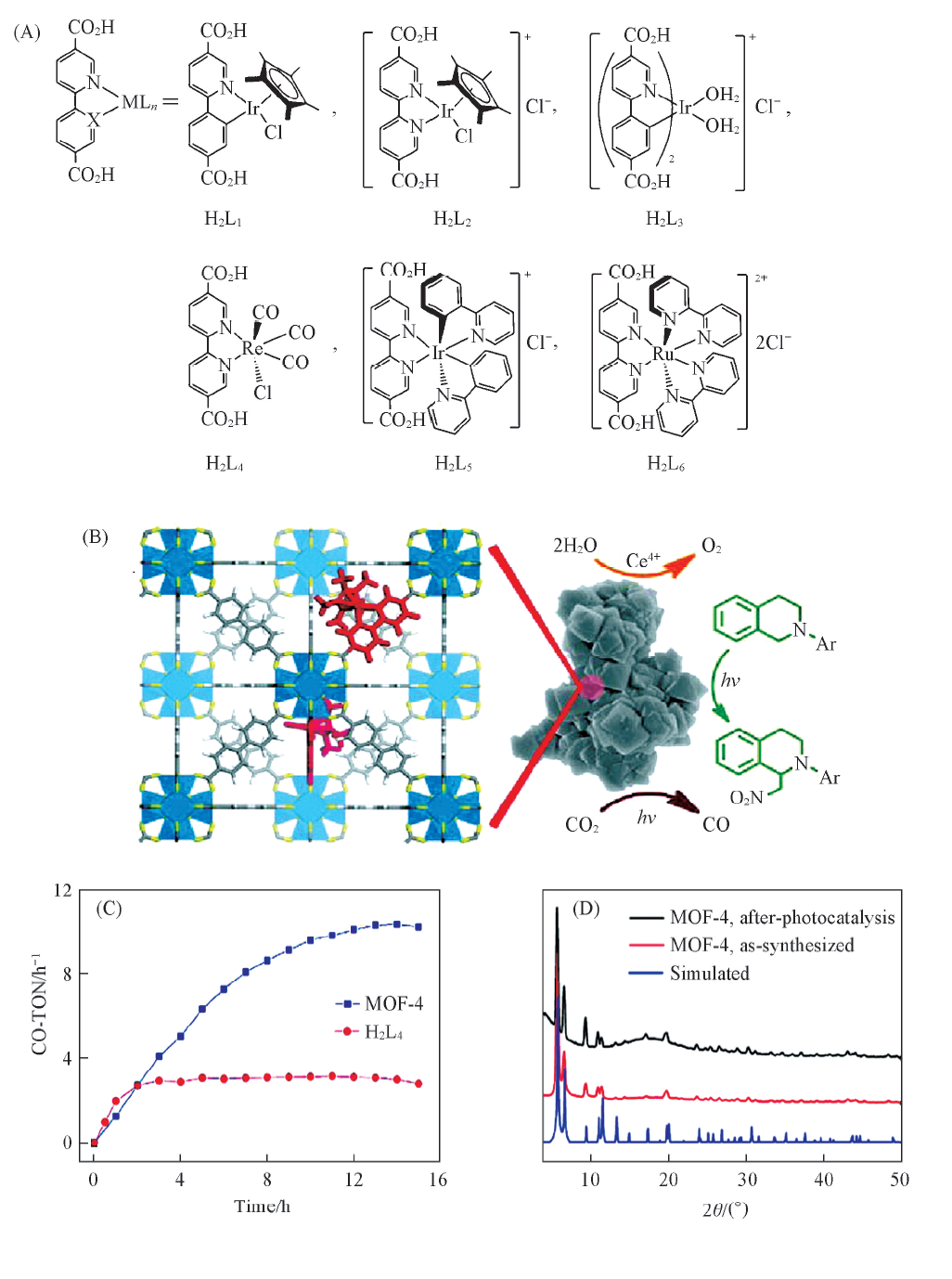
Fig.2 Ligand structure of H2L1-H2L6(A), photocatalytic conversion schematic(B), plots of CO-TON versus time(C) and PXRD patterns of the catalysts(D)[41] Copyright 2011, American Chemical Society.
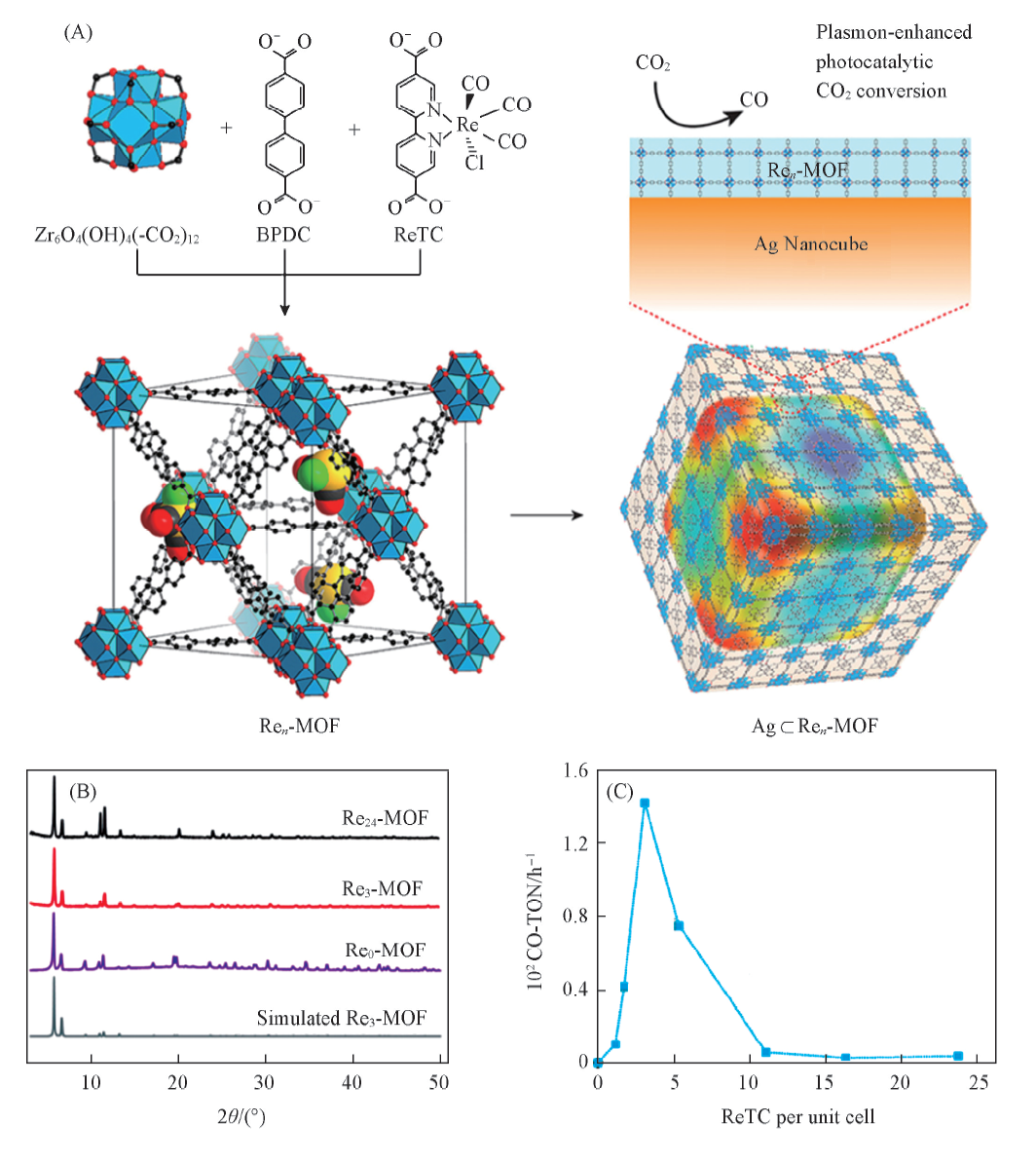
Fig.3 Structures of Ren-MOF and Ag?Ren-MOF based catalysts(A), PXRD of Ren-MOFs(B) and the photocatalytic activity of Ren-MOF(C)[42] Copyright 2017, American Chemical Society.
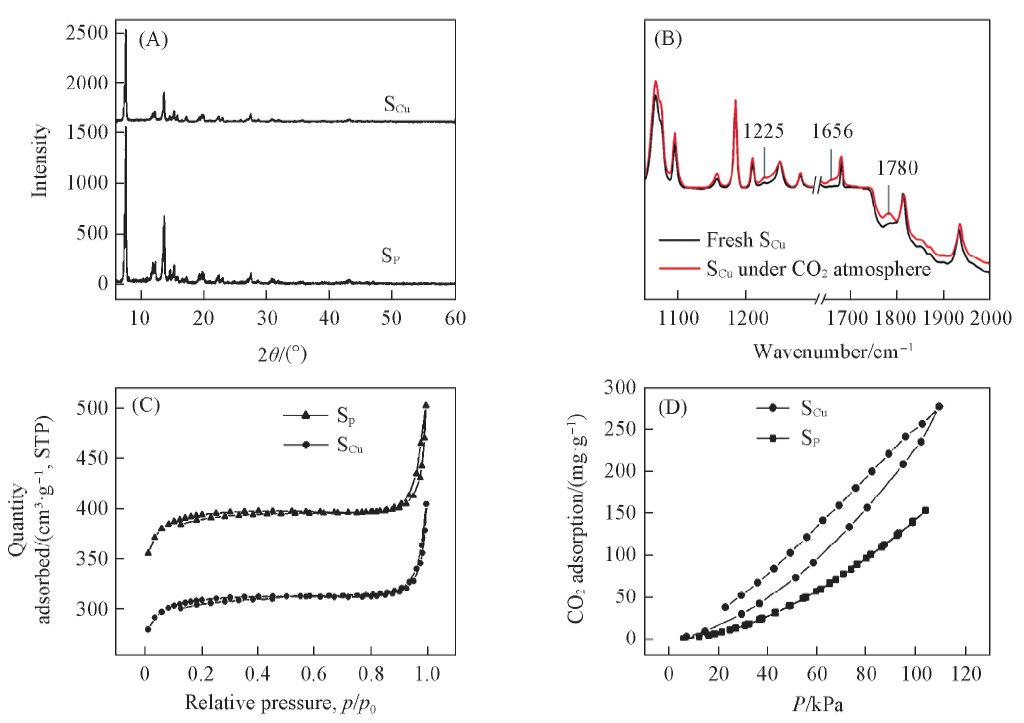
Fig.4 XRD patterns(A) and FTIR spectra(B), N2(C) and CO2(D) adsorption and desorption isotherms of SCu and SP[43] Copyright 2013, American Chemical Society.
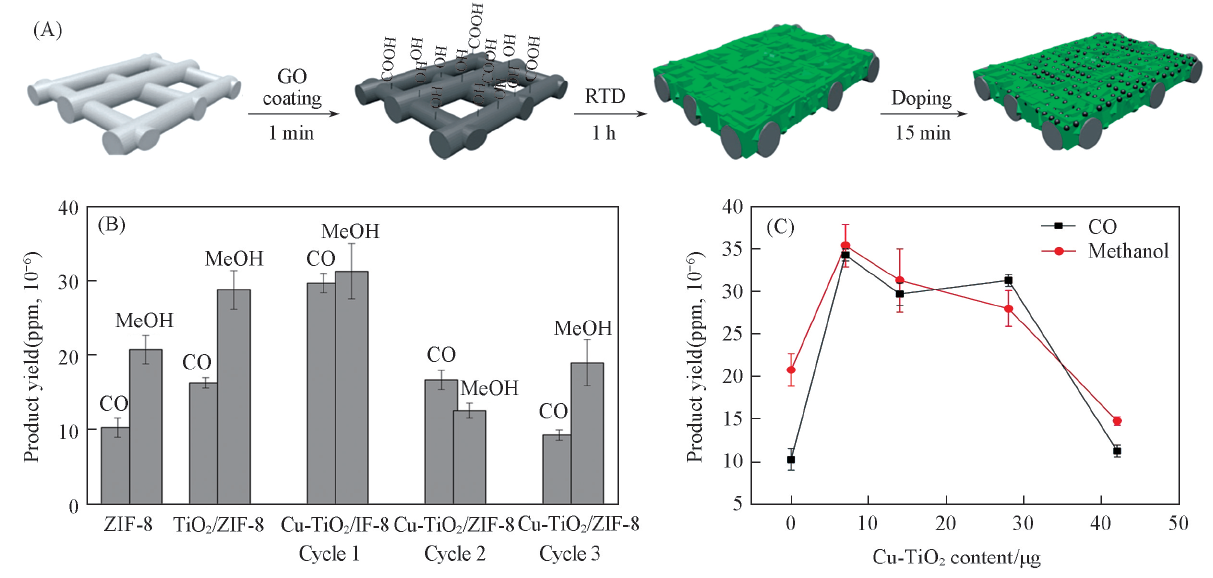
Fig.5 Fabrication of Cu-TiO2/ZIF-8 membranes(A), effect of membrane composition(B) and Cu-TiO2 nanoparticles loading on the product yields(C)[51] Copyright 2017, American Chemical Society.
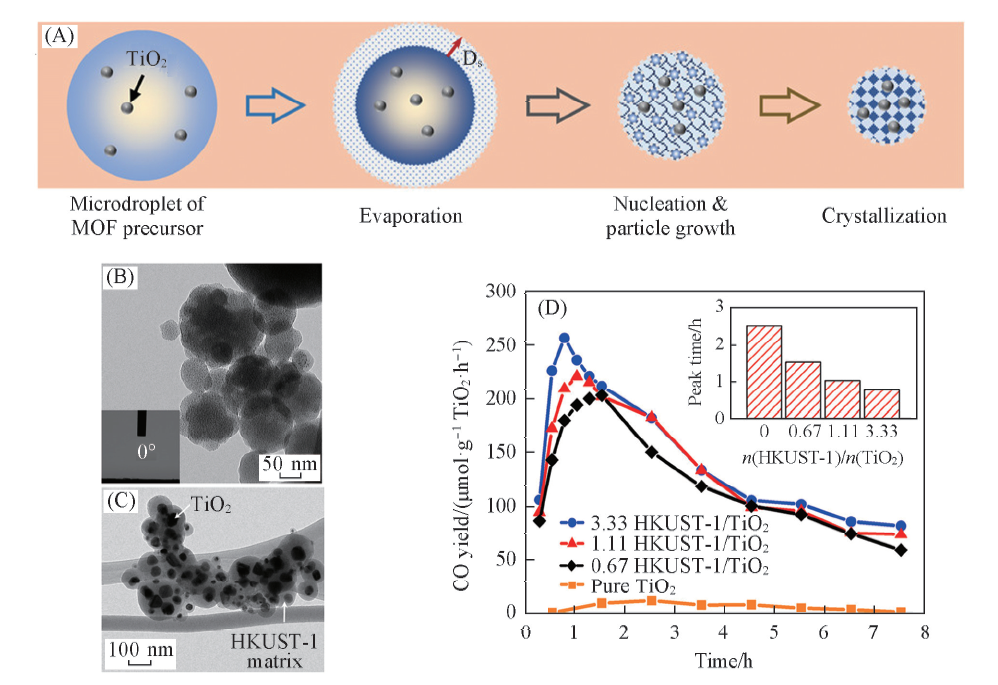
Fig.6 HKUST-1 and HKUST-1/TiO2 formation steps inside a microdroplet(A), TEM images of as-synthesized HKUST-1(B) and 33.3 HKUST-1/TiO2(C) at 300 ℃ and CO2 photoreduction performance of TiO2 and HKUST-1/TiO2 composites(D)[52] Inset of (B): the image of the contact angle measurement of HKUST-1 surface. Copyright 2017, American Chemical Society.
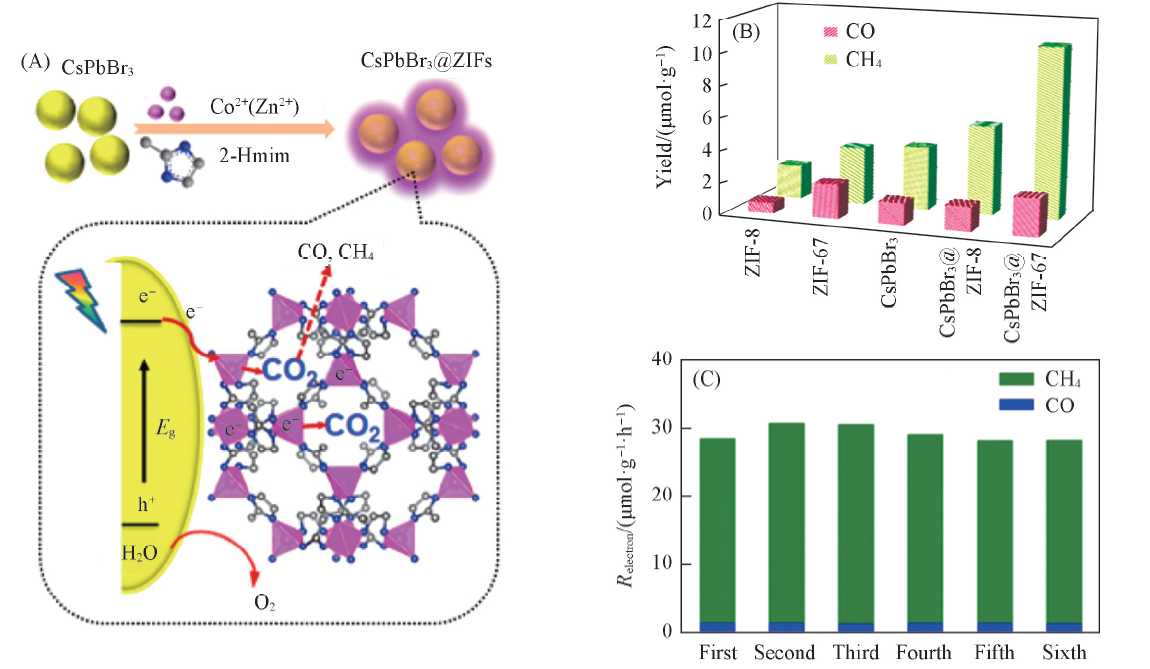
Fig.7 Schematic illustration of the fabrication process and CO2 photoreduction process of CsPbBr3/ZIFs(A) and photocatalytic CO2 reduction performances of CsPbBr3 and CsPbBr3@ZIFs(B, C)[59] Copyright 2018, American Chemical Society.
| [1] | Klankermayer J., Wesselbaum S., Beydoun K., Leitner W., Angew. Chem. Int. Ed.,2016, 55(26), 7267—7343 |
| [2] | Beckman E. J., Nature, 2016, 531(7593), 180—181 |
| [3] | He M. Y., Sun Y. H., Han B. X., Angew. Chem. Int. Ed.,2013, 52(37), 9620—9633 |
| [4] | Beckman E. J., Environ. Sci.Technol., 2002, 36(17), 347—353 |
| [5] | Markewitz P., Kuckshinrichs W., Leitner W., Linssen J., Zapp P., Bongartz R., Schreiber A., Müller T. E., Energ. Environ. Sci.,2012, 5(6), 7281—7305 |
| [6] | Hong J. D., Zhang W., Ren J., Xu R., Anal. Methods,2013, 5(5), 1086—1097 |
| [7] | Liu X., Inagaki S., Gong J., Angew. Chem. Int. Ed.,2016, 55(48), 14924—14950 |
| [8] | Wang S., Wang X., Angew. Chem. Int. Ed.,2016, 55(7), 2308—2320 |
| [9] | Liu M., Pang Y. J., Zhang B., Luna P. D., Voznyy O., Xu J. X., Zheng X. L., Nature,2016, 537(7620), 382—386 |
| [10] | Gao S., Lin Y., Jiao X. C., Sun Y. F., Luo Q. Q., Zhang W. H., Li D. Q., Yang J. L., Xie Y., Nature,2016, 529(7584), 68—71 |
| [11] | Bailleul B., Berne N., Murik O., Petroutsos D., Prihoda J., Tanaka A., Villanova V., Bligny R., Flori S., Falconet D., Krieger-Liszkay A., Santabarbara S., Rappaport F., Joliot P., Tirichine L., Falkowski P. G., Cardol P., Bowler C., Finazzi G., Nature,2015, 524(7565), 366—369 |
| [12] | Mifsud M., Gargiulo S., Iborra S., Arends I. W. C. E., Hollmann F., Corma A., Nat. Commun.,2014, 5, 3145 |
| [13] | Shi J., Jiang Y., Jiang Z., Wang X., Wang X., Zhang S., Han P., Yang C., Chem. Soc. Rev.,2015, 44(17), 5981—6000 |
| [14] | Zhou Y., Zhang L., Lin L., Wygant B. R., Liu Y., Zhu Y., Zheng Y., Mullins C. B., Zhao Y., Zhang X., Yu G., Nano Lett.,2017, 17(12), 8012—8017 |
| [15] | Thompson W. A., Perier C., Maroto-Valer M. M., Appl. Catal. B: Environ.,2018, 238, 136—146 |
| [16] | Jang Y. J., Jang J. W., Lee J., Kim J. H., Kumagai H., Lee J., Minegishi T., Kubota J., Domen D., Lee J. S., Energy Environ. Sci.,2015, 8(12), 3597—3604 |
| [17] | Kumar P., Joshi C., Barras A., Sieber B., Addad A., Boussekey L., Szunerits S., Boukherroub R., Jain S. L., Appl. Catal. B: Environ.,2017, 205, 654—665 |
| [18] | Zheng Z. Z., Xu H. T., Xu Z. L., Ge J. P., Small,2018, 14(5), 1702812 |
| [19] | Pitre S. P., Mctiernan C. D, Vine W., DiPucchio R., Grenier M., Scaiano J. C., Sci. Rep.,2015, 5, 16397 |
| [20] | Li Q., Guo B. D., Yu J. G., Ran J. G., Zhang B. H., Yan H. J., Gong J. R., J. Am. Chem. Soc.,2011, 133(28), 10878—10884 |
| [21] | Liu Y. N., Wang R. X., Yang Z. K., Du H., Jiang Y. F., Shen C. C., Liang K., Xu A. W., Chinese J. Catal.,2015, 36(12), 2135—2144 |
| [22] | Li X., Yu J., Low J., Fang Y. P., Xiao J., Chen X. B., J. Mater. Chem. A,2015, 3(6), 2485—2534 |
| [23] | Kuppler R. J., Timmons D. J., Fang Q. R., Li J. R., Makal T. A., Young M. D., Yuan D., Zhao D., Zhuang W., Zhou H. C., Coordin. Chem. Rev.,2009, 253(23/24), 3042—3066 |
| [24] | Li J. R., Sculley J., Zhou H. C., Chem. Rev.,2011, 112(2), 869—932 |
| [25] | Li J. R., Yu J., Lu W., Sun L. B., Sculley J., Balbuena P. B., Zhou H. C., Nat. Commun.,2013, 4, 1538 |
| [26] | Lan M., Guo R. M., Dou Y. B., Zhou J., Zhou A. W., Li J. R., Nano Energy,2017, 33, 238—246 |
| [27] | Dou Y. B., Zhou J., Zhou A W., Li J. R., Nie Z. R., J. Mater. Chem. A,2017, 5(36), 19491—19498 |
| [28] | Sumida K., Rogow D. L., Mason J. A., McDonald T. M., Bloch E. D., Herm Z. R., Bae T. H., Long J. R., Chem. Rev.,2012, 112(2), 724—781 |
| [29] | Yang H., Du R. F., Fu Y. H., Guangdong Chemical Industry,2017, 44(10), 97—98 |
| (杨欢, 杜荣飞, 傅仰河. 广东化工, 44(10), 97—98) | |
| [30] | Lee C. Y., Farha O. K., Hong B. J., Sarjeant A. A., Nguyen S. T., Hupp J. T., J. Am. Chem. Soc.,2011, 133(40), 15858—15861 |
| [31] | Luo T., Zhang J. L., Li W., He Z. H., Sun X. F., Shi J. B., Shao D., Zhang B. X., Tan X. N., Han B. X., ACS Appl. Mater. Interfaces,2017, 9 (47), 41594—41598 |
| [32] | Chen D. S., Xing H. Z., Wang C. Q., Su Z. M., J. Mater. Chem. A,2016, 4(7), 2657—2662 |
| [33] | Xu H. Q., Hu J. H., Wang D. K., Li Z. H., Zhang Q., Luo Y., Yu S. H., Jiang H. L., J. Am. Chem. Soc.,2015, 137(42), 13440—13443 |
| [34] | Sadeghi N., Sharifnia S., Arabi M. S., J. CO2 Util. ,2016, 16, 450—457 |
| [35] | Costentin C., Drouet S., Robert M., Saveant J. M., Science,2012, 338, 90—94 |
| [36] | Wang D., Huang R., Liu W., Li Z., ACS Catal.,2014, 4(12), 4254—4260 |
| [37] | Zhang S. Q., Li L., Zhao S. G., Sun Z. H., Hong M. C., Luo J. H., J. Mater. Chem. A,2015, 3(30), 15764—15768 |
| [38] | Dan-Hardi M., Serre C., Frot T., Rozes L., Maurin G., Sanchez C., Férey G., J. Am. Chem. Soc.,2010, 131(31), 10857—10859 |
| [39] | Fu Y., Sun D., Chen Y., Huang R., Ding Z., Fu X., Li Z., Angew. Chem. Int. Ed.,2012, 51(14), 3364—3367 |
| [40] | Sun D. R., Fu Y. H., Liu W. J., Ye L., Wang D. K., Yang L., Fu X. Z., Li Z. H., Chem. Eur. J.,2013, 19(42), 14279—14285 |
| [41] | Wang C., Xie Z., Dekrafft K. E., Lin W., J. Am. Chem. Soc.,2011, 133(34), 13445—13454 |
| [42] | Choi K. M., Kim D., Rungtaweevoranit B., Trickett C. A., Barmanbek J. T., Alshammari A. S., Yang P., Yaghi O. M., J. Am. Chem. Soc.,2017, 139(1), 356—362 |
| [43] | Liu Y., Yang Y., Sun Q., Wang Z., Huang B., Dai Y., Qin X., Zhang X., ACS Appl. Mater. Interface,2013, 5(15), 7654—7658 |
| [44] | Fei H., Sampson M. D., Lee Y., Kubiak C. P., Cohen S. M., Inorg. Chem.,2015, 54(14), 6821—6828 |
| [45] | Lee Y., Kim S., Fei H., Kang J. K., Cohen S. M., Chem. Commun.,2015, 51(92), 16549—16552 |
| [46] | Chen Y., Wang D., Deng X., Li Z. H., Catal. Sci. Technol.,2017, 7(21), 4893—4904 |
| [47] | Yui T., Kan A., Saitoh C., Koike K., Ibusuki T., Ishitani O., ACS Appl. Mater. Interfaces,2011, 3(7), 2594—2600 |
| [48] | Ola O., Valer M. M., Liu D., Mackintosh S., Lee C. W., Wu J. C. S., Appl. Catal. B,2012, 126, 172—179 |
| [49] | Habisreutinger S. N., Schmidt-Mende L., Stolarczyk J. K., Angew. Chem. Int. Ed.,2013, 52(29), 7372—7408 |
| [50] | Yan S. S., Ouyang S. X., Xu H., Zhao M., Zhang X. L., Ye J. H., J. Mater. Chem. A,2016, 4(39), 15126—15133 |
| [51] | Maina J. W., Schütz J. A., Grundy L., Ligneris E. D., Yi Z. F., Kong L. X., Pozo-Gonzalo C., Ionescu M., L. F., ACS Appl. Mater. Interface,2017, 9(40), 35010—35017 |
| [52] | He X., Gan Z., Fisenko S., Wang D., El-Kaderi H. M., Wang W. N., ACS Appl. Mater. Interface,2017, 9(11), 9688—9698 |
| [53] | Liu Q., Low Z. X., Li L. X., Razmjoua A., Wang K., Yao J. F., Wang H. T., J. Mater. Chem. A,2013, 1(38), 11563—11569 |
| [54] | Zheng Y., Lin L., Ye X., Guo F., Wang X., Angew. Chem. Int. Ed.,2014, 53(44), 11926—11930 |
| [55] | Lin J., Pan Z., Wang X., ACS Sustainable Chem. Eng.,2014, 2(3), 353—358 |
| [56] | Wang W., Yu J. C., Shen Z., Chan D. K., Gu T., Chem. Commum.,2014, 50(70), 10148—10150 |
| [57] | Liu S., Chen F., Li S., Xiong Y., Appl. Catal. B: Environ.,2017, 211, 1—10 |
| [58] | Li R., Hu J., Deng M., Wang H., Wang X., Hu Y., Jiang H. L., Jiang J., Zhang Q., Xie Y., Xiong Y., Adv. Mater.,2014, 26(28), 4783—4788 |
| [59] | Kong Z. C., Liao J. F., Dong Y. J., Xu Y. F., Chen H. C., Kuang D. B., Su C., Y., ACS Energy Lett.,2018, 3(11), 2656—2662 |
| [60] | Su Y., Zhang Z., Liu H., Wang W., Appl. Catal. B: Environ.,2017, 200, 448—457 |
| [61] | Sun D., Gao Y., Fu J., Zeng X., Chen Z., Li Z., Chem. Commun.,2015, 51(13), 2645—2648 |
| [62] | Qin J., Wang S., Wang X., Appl. Catal. B: Environ.,2017, 209, 476—482 |
| [63] | Shi L., Wang T., Zhang H., Chang K., Ye J., Adv. Funct. Mater.,2015, 25(33), 5360—5367 |
| [64] | Zhang H., Wei J., Dong J., Liu G., Shi L., An P., Zhao G., Kong J., Wang X., Meng X., Zhang J., Ye J., Angew. Chem. Int. Ed.,2016, 55(46), 14310—14314 |
| [1] | 吴玉, 李轩, 杨恒攀, 何传新. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9): 20220343. |
| [2] | 王新天, 李攀, 曹越, 洪文浩, 耿忠璇, 安志洋, 王昊宇, 王桦, 孙斌, 朱文磊, 周旸. 单原子材料在二氧化碳催化中的技术经济分析与产业化应用前景[J]. 高等学校化学学报, 2022, 43(9): 20220347. |
| [3] | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 20220300. |
| [4] | 何鸿锐, 夏文生, 张庆红, 万惠霖. 羟基氧化铟团簇与二氧化碳和甲烷作用的密度泛函理论研究[J]. 高等学校化学学报, 2022, 43(8): 20220196. |
| [5] | 崔伟, 赵德银, 白文轩, 张晓东, 余江. CO2在非质子溶剂与铁基离子液体复合体系中的吸收[J]. 高等学校化学学报, 2022, 43(8): 20220120. |
| [6] | 郭志强, 杨博如, 席婵娟. 硼氢化试剂在二氧化碳还原官能化反应中的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220199. |
| [7] | 张昕昕, 许狄, 王艳秋, 洪昕林, 刘国亮, 杨恒权. CO2加氢制低碳醇CuFe基催化剂中的Mn助剂效应[J]. 高等学校化学学报, 2022, 43(7): 20220187. |
| [8] | 周紫璇, 杨海艳, 孙予罕, 高鹏. 二氧化碳加氢制甲醇多相催化剂研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220235. |
| [9] | 王征文, 高凤翔, 曹瀚, 刘顺杰, 王献红, 王佛松. 基于二氧化碳共聚物的紫外光固化高分子材料的制备与性能[J]. 高等学校化学学报, 2022, 43(7): 20220236. |
| [10] | 黄孝舜, 马海英, 柳淑娟, 王斌, 王红利, 钱波, 崔新江, 石峰. 二氧化碳间接转化制化学品的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220222. |
| [11] | 宋德文, 汪明旺, 王亚旎, 焦振梅, 宁汇, 吴明铂. 二氧化碳电还原制草酸研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220248. |
| [12] | 赵润瑶, 纪桂鹏, 刘志敏. 吡咯氮配位单原子铜催化剂的电催化二氧化碳还原性能[J]. 高等学校化学学报, 2022, 43(7): 20220272. |
| [13] | 邱丽琪, 姚向阳, 何良年. 可见光驱动丰产金属卟啉类配合物催化的二氧化碳选择性还原反应[J]. 高等学校化学学报, 2022, 43(7): 20220064. |
| [14] | 彭奎霖, 李桂林, 江重阳, 曾少娟, 张香平. 电解液调控CO2电催化还原性能微观机制的研究进展[J]. 高等学校化学学报, 2022, 43(7): 20220238. |
| [15] | 张振, 邓煜, 张琴芳, 余达刚. 可见光促进二氧化碳参与的羧基化反应[J]. 高等学校化学学报, 2022, 43(7): 20220255. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 1861
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 2206
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||