Electrochemical properties of outer-sphere associates of bipyridyl and sepulchrate metal complexes with (thia)calix[4]arenes |
| |
| Authors: | Alexey Stepanov Vitaly Yanilkin Asiya Mustafina Svetlana Solovieva |
| |
| Institution: | 1.Arbuzov Institute of Organic and Physical Chemistry,FRC Kazan Scientific Center of RAS,Kazan,Russia |
| |
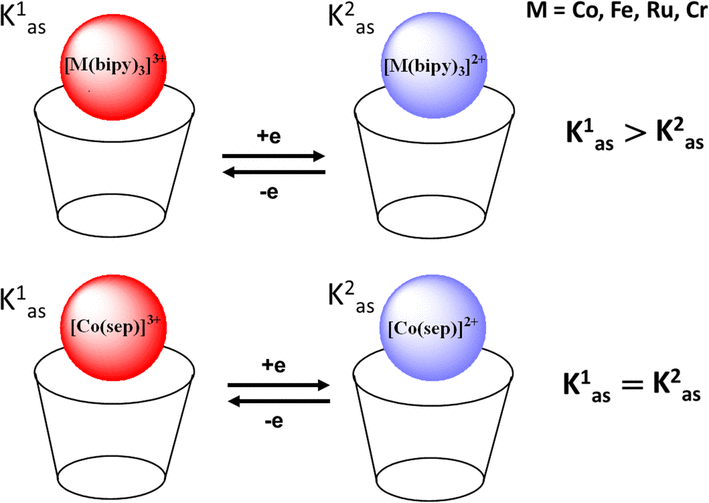
| Abstract: | The electrochemical one-electron reduction (oxidation) of bipyridyl metal complexes (Co(bipy)3]3+, Cr(bipy)3]3+, Fe(bipy)3]2+, Ru(bipy)3]2+ (as well as Co(III) sepulcrate)) with water-soluble (thia)calix4]arenes has been studied by means of cyclic voltammetry. It has been shown that M(bipy)3]3+/2+ bind to (thia)calix4]arenes via sulfonate groups of the upper rim. Oxidized forms bind stronger than reduced ones leading to reduction (oxidation) of half-wave cathodic shift. The effect of predominant stabilization of oxidized forms of metal complexes for carboxylated calix4]arene is stronger than for thiacalix4]arene (ΔΔG0?=???7.8?÷???12.5 and ??3.7 kJ/mol, respectively). The redox-switchable outer-sphere binding of Co(III) sepulchrate via lower rim of carboxylated calix4]arene has been revealed using cyclic voltammetry. The binding constants of outer-sphere associates based on calix4]arenes and unstable metal complexes (Co(sep)]2+, Ru(bipy)3]3+, Co(bipy)3]2+) have been calculated for the first time using 1H NMR titration and cyclic voltammetry data. | |
| Keywords: | |
| 本文献已被 SpringerLink 等数据库收录! |
|