Preparation of a new FerroPHOS derivative for palladium-catalyzed asymmetric allylic alkylations |
| |
| Affiliation: | 1. Department of Chemistry, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, USA;2. Department of Medicinal Chemistry, University of Minnesota, 308 Harvard Street SE, Minneapolis, MN 55455, USA |
| |
| Abstract: | 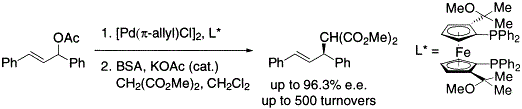
A new C2-symmetric only cylindrically chiral FerroPHOS derivative possessing (1-methoxy-1-methyl)ethyl substituents was prepared and applied to the palladium-catalyzed asymmetric allylic alkylation of 1,3-diphenylprop-2-en-1-yl acetate. Both high catalytic activity (up to 500 turnovers) and enantioselectivity (e.e.'s of up to 96.3%) were attained in reactions using the new diphosphine ligand. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|