Crystal structure and heat capacity of the mixed-valence trinuclear iron monoiodoacetate complex, [Fe(III)2Fe(II)O(O2CCH2I)6(H2O)3][Fe(III)3O(O2CCH2I)6(H2O)3]I |
| |
| Affiliation: | 1. University at Albany, SUNY, 1400 Washington Avenue, Albany, NY 12222, USA;2. Gensoric GmbH, G.-Hauptmann-Str. 23, 18055 Rostock, Germany;1. Division of Rheumatology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan;2. Division of Rheumatology, Showa University Kototoyosu Hospital, Tokyo, Japan |
| |
| Abstract: | 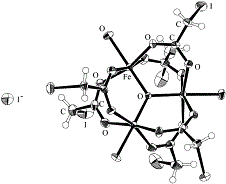
The crystal structure of a trinuclear iron monoiodoacetate complex was determined. Although it has been incorrectly characterized as [Fe3O(O2CCH2I)6(H2O)3], the correct chemical formula turned out to be [Fe(III)2Fe(II)O(O2CCH2I)6(H2O)3]-[Fe(III)3O(O2CCH2I)6(H2O)3]I (1). The two kinds of Fe3O molecules (Fe(III)2Fe(II)O and Fe(III)3O) are crystallographically indistinguishable. All the Fe atoms are crystallographically equivalent because of a crystallographic threefold symmetry. Heat capacities of 1 seem to exhibit no thermal anomaly in the temperature range 5.5–309 K, although the valence detrapping phenomenon has been observed in this temperature range. This fact indicates that the valence-detrapping phenomenon in 1 occurs without any phase transition, leading 1 to a glassy state, probably because the crystal of 1 is just like a solid solution of distorted mixed-valence Fe(III)2Fe(II)O molecules and permanently undistorted Fe(III)3O molecules which may act as an inhibitor for a cooperative valence-trapping. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

