Univocal syntheses of 2- and 3-hydroxymethyl-2,3-dihydro[1,4]dioxino[2,3-b]pyridine enantiomers |
| |
| Affiliation: | 1. Dipartimento di Scienze Farmaceutiche, Università degli Studi di Milano, Via Mangiagalli 25, 20133 Milano, Italy;2. Dipartimento di Scienze Biomediche Chirurgiche e Odontoiatriche, Università degli Studi di Milano, Via C. Pascal 36, 20133 Milano, Italy;3. Dipartimento di Scienze Farmacologiche e Biomolecolari—DiSFeb, Università degli Studi di Milano, via C. Pascal 36, 20133 Milano, Italy;4. Dipartimento di Farmacia, Università di Genova, Viale Benedetto XV 3, 16132 Genova, Italy;1. Institut für Pharmazeutische und Medizinische Chemie der Westfälischen Wilhelms-Universität Münster, Corrensstraße 48, D-48149, Münster, Germany;2. Cells-in-Motion Cluster of Excellence (EXC 1003 – CiM), Westfälische Wilhelms-Universität Münster, Germany;1. Department of Medicinal Chemistry, Amgen Inc., 360 Binney Street, Cambridge, MA 02142, USA;2. Department of Discovery Chemistry, Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, MA 02115, USA;1. School of Pharmacy, Showa University, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan;2. Nihon Pharmaceutical University, 10281 Komuro, Inamachi, Kita-adachi-gun, Saitama 362-0806, Japan;3. Yokohama College of Pharmacy, 601 Matano-cho, Totsuka-ku, Yokohama-shi, Kanagawa 245-0066, Japan;4. Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-12, Nishi-6, Kita-ku, Sapporo 060-0812, Japan;5. Department of Tumor Virology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Okayama 700-8558, Japan;6. Rega Institute for Medical Research, Katholieke Universiteit Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium;1. Jiangxi Key Laboratory of Organic Chemistry, Jiangxi Science and Technology Normal University, 605 Fenglin Avenue, Nanchang, Jiangxi 330013, PR China;2. High Level Engineering Research Center of Biopharmaceutical Molecules and Diagnostic Apparatuses, Jiangxi Provincial Colleges and Universities, Nanchang, Jiangxi 330013, PR China |
| |
| Abstract: | 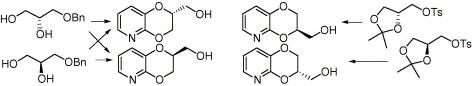
The enantiomers of 2- and 3-hydroxymethyl substituted 2,3-dihydro[1,4]dioxino[2,3-b]pyridine 1 and 2, important chiral building blocks for the preparation of several biologically active compounds, were synthesized. (S)- and (R)-1 were obtained from either one or both the enantiomers of benzylglycerol, while (S)- and (R)-2 were obtained from (R)- and (S)-isopropylideneglycerol, respectively. The novel efficient synthetic strategies, which do not follow routes already reported for the corresponding racemates, ensure very high regioselectivity and maintenance of the enantiomeric purity of the starting materials. The enantiomeric composition of the title compounds was determined by chiral HPLC or NMR. The key intermediate in the synthesis of non-racemic 1, namely 1-benzyl-2-mesyl-3-tritylglycerol, is a new high melting chiral C3 synthon, worth considering for its stability, versatility, easy isolation by simple crystallization and, potential of configuration inversion through a simple one-pot reaction sequence. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

