Selective sialylation of 2,3-di-O-(β-d-galactopyranosyl)-d-galactose catalyzed by Trypanosoma cruzi trans-sialidase |
| |
| Affiliation: | 1. Laboratory of Natural Products, Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia;2. Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia;3. Drug and Herbal Research Centre, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abd. Aziz, 50300 Kuala Lumpur, Malaysia;4. Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia;1. Department of Organic Chemistry, Faculty of Chemistry, Nicolaus Copernicus University in Torun, 7 Gagarin Street, 87-100 Torun, Poland;2. Department of Crystallochemistry and Biocrystallography, Faculty of Chemistry, Nicolaus Copernicus University in Torun, 7 Gagarin Street, 87-100 Torun, Poland;1. Instituto de Química del Sur, INQUISUR (CONICET-UNS), Departamento de Química, Universidad Nacional del Sur, Av. Alem 1253, 8000 Bahía Blanca, Argentina;2. Planta Piloto de Ingeniería Química, PLAPIQUI (CONICET-UNS), Camino La Carrindanga Km 7, CC 717, 8000 Bahía Blanca, Argentina;1. Institute of Biological Chemistry, Academia Sinica, No 128, Sec 2, Academia Rd, Nankang, Taipei 11529, Taiwan;2. Institute of Physics, Academia Sinica, Taipei 11529, Taiwan;3. Department of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan;4. Institute of Biochemical Sciences, National Taiwan University, Taipei 10617, Taiwan;1. Laboratorio de Biología del Cáncer, Instituto de Investigaciones Bioquímicas Bahía Blanca, Centro Científico Tecnológico Bahía Blanca (INIBIBB-CONICET), Bahía Blanca, Argentina;2. Laboratorio de Química Orgánica, Departamento de Química, Universidad Nacional del Sur (INQUISUR), Bahía Blanca, Argentina;3. Departamento de Química Orgánica, Facultad de Química and Instituto de Investigación Biomedica (IBI), University of Vigo, Campus Lagoas de Marcosende, 36310 Vigo, Spain;4. Area de Investigación, Instituto de Oncología “Angel H. Roffo”, Buenos Aires, Argentina;1. Division of Pharmacology, Meikai University School of Dentistry, Sakado, Saitama, Japan;2. Department of Electron Microscope, Meikai University School of Dentistry, Sakado, Saitama, Japan;3. Division of Anatomy, Meikai University School of Dentistry, Sakado, Saitama, Japan;4. Institute for Advanced Bioscience, Keio University, Tsuruoka, Yamagata, Japan;5. Department of Biochemistry, Faculty of Pharmaceutical Sciences, Tokyo University of Science, Noda, Chiba, Japan;6. Faculty of Pharmaceutical Sciences, Josai University, Sakado, Saitama, Japan |
| |
| Abstract: | 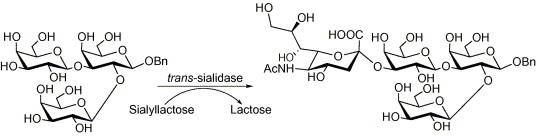
Trypanosoma cruzi, the agent of Chagas’ disease, expresses on its surface a trans-sialidase (TcTS) that transfers sialic acid from host glycoconjugates to terminal β-galactopyranosyl units of parasite mucins. This process is involved in infection and pathogenesis. The trisaccharide 2,3-di-O-(β-d-galactopyranosyl)-d-galactose 1 is an external unit in the larger oligosaccharides of the mucins and a site for sialylation. The trisaccharide was previously synthesized in our laboratory. The last step of the synthesis was the hydrogenolysis of the crystalline benzyl trisaccharide. Herein we prove that the trisaccharide 1, its alditol 3 and the benzyl glycoside 2 are good acceptors of sialic acid and effective inhibitors of the sialylation of N-acetyllactosamine catalyzed by TcTS. Furthermore, selective sialylation of the 1→3 linked galactopyranose in glycoside 2 was determined by one and two-dimensional NMR analysis. In contrast, the flexible 2,3-di-O-(β-d-galactopyranosyl)-d-galactitol 3 is sialylated in either one of the two possible sites. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

