One-step hydrothermal synthesis of LiMn2O4 cathode materials for rechargeable lithium batteries |
| |
| Affiliation: | 1. Department of Advanced Materials, College of Materials Science and Engineering, Sichuan University, Chengdu, 610065, China;2. Institute of New Energy and Low-carbon Technology, Sichuan University, Chengdu, 610065, China;1. Department of Chemistry, College of Sciences, Shanghai University, Shanghai 200444, PR China;2. Department of Physics, College of Sciences, Shanghai University, Shanghai 200444, PR China;1. National Center for Nanoscience and Technology of China, Beijing 100190, PR China;2. Department of Physics, Tsinghua University, Beijing 100080, PR China;1. School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, Henan, PR China;2. National Research Council of Canada, Vancouver, BC, Canada V6T IW5;1. State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry & Chemical Engineering, Nanjing Tech University, Number 5 Xin Mofan Road, Nanjing, Jiangsu 210009, People''s Republic of China;2. Department of Chemical Engineering, Curtin University, Perth, Western Australia 6845, Australia;1. School of Chemical Machinery, Dalian University of Technology, Dalian 116024, China;2. Center for Green Products and Processing Technologies, Guangzhou HKUST Fok Ying Tung Research Institute, Guangzhou 511458, China;3. School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China;4. Department of Chemical and Biomolecular Engineering, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China |
| |
| Abstract: | 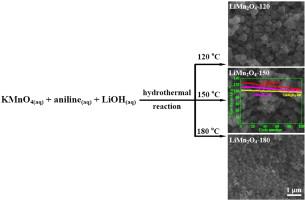
LiMn2O4 cathode materials with high discharge capacity and good cyclic stability were prepared by a simple one-step hydrothermal treatment of KMnO4, aniline and LiOH solutions at 120–180 °C for 24 h. The aniline/KMnO4 molar ratio (R) and hydrothermal temperature exhibited an obvious influence on the component and phase structures of the resulting product. The precursor KMnO4 was firstly reduced to birnessite when R was less than 0.2:1 at 120–150 °C. Pure-phased LiMn2O4 was formed when R was 0.2:1, and the LiMn2O4 was further reduced to Mn3O4 when R was kept in the range of 0.2–0.3 at 120–150 °C. Moreover, LiMn2O4 was fabricated when R was 0.15:1 at 180 °C. Octahedron-like LiMn2O4 about 300 nm was prepared at 120 °C, and particle size decreased with an increase in hydrothermal temperature. Especially, LiMn2O4 synthesized at 150 °C exhibited the best electrochemical performance with the highest initial discharge capacity of 127.4 mAh g−1 and cycling capacity of 106.1 mAh g−1 after 100 cycles. The high discharge capacity and cycling stability of the as-prepared LiMn2O4 cathode for rechargeable lithium batteries were ascribed to the appropriate particle size and larger cell volume. |
| |
| Keywords: | Hydrothermal reaction Crystal growth Particle size Electrochemical properties |
| 本文献已被 ScienceDirect 等数据库收录! |
|

