Structure formation due to antagonistic salts |
| |
| Affiliation: | 1. Department of Physics, Kyoto University, Kyoto 606-8502, Japan;2. Yukawa Institute for Theoretical Physics, Kyoto University, Kyoto 606-8502, Japan;3. Department of Chemistry, Tokyo Metropolitan University, Hachioji, Tokyo 192-0397, Japan |
| |
| Abstract: | 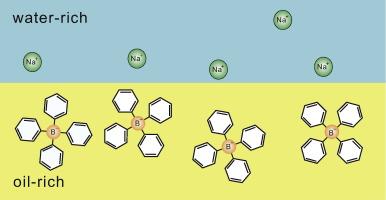
Antagonistic salts are composed of hydrophilic and hydrophobic ions. In a mixture solvent (water–oil) such ion pairs are preferentially attracted to water or oil, giving rise to a coupling between the charge density and the composition. First, they form a large electric double layer at a water–oil interface, reducing the surface tension and producing mesophases. Here, the cations and anions are loosely bound by the Coulomb attraction across the interface on the scale of the Debye screening length. Second, on solid surfaces, hydrophilic (hydrophobic) ions are trapped in a water-rich (oil-rich) adsorption layer, while those of the other species are expelled from the layer. This yields a solvation mechanism of local charge separation near a solid. In particular, near the solvent criticality, disturbances around solid surfaces can become oscillatory in space. In mesophases, we calculate periodic structures, which resemble those in experiments. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

