The physics of protein self-assembly |
| |
| Affiliation: | 1. Department of Chemistry, Maynooth University, Maynooth, Co. Kildare, Ireland;2. Department of Chemistry, Duke University, Durham, NC, USA;3. CNR ISC, UOS Sapienza, Piazzale A. Moro 5, 00185 Rome, Italy;4. Department of Physics, Sapienza University of Rome, Piazzale A. Moro 5, 00185 Rome, Italy;5. Department of Physics, Yeshiva University, New York, NY, USA;6. Department of Biology, Yeshiva University, New York, NY, USA |
| |
| Abstract: | 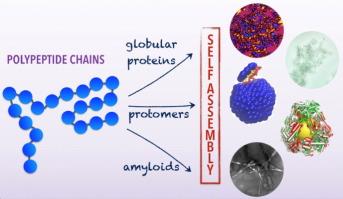
Understanding protein self-assembly is important for many biological and industrial processes. Proteins can self-assemble into crystals, filaments, gels, and other amorphous aggregates. The final forms include virus capsids and condensed phases associated with diseases such as amyloid fibrils. Although seemingly different, these assemblies all originate from fundamental protein interactions and are driven by similar thermodynamic and kinetic factors. Here we review recent advances in understanding protein self-assembly through a soft condensed matter perspective with an emphasis on three specific systems: globular proteins, viruses, and amyloid fibrils. We conclude with a discussion of unanswered questions in the field. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

