Standard molar volumes and expansibilities of 1,3-alkyl-N-substituted achiral glycolurils in water at T = (278.15 to 318.15) K and p = 0.1 MPa: A comparative analysis |
| |
| Affiliation: | 1. Hospital General “Dr. Manuel Gea Gonzalez”, Mexico City, Mexico;2. Laboratorio de Entomologia, Escuela Nacional de Ciencias Biologicas, Instituto Politecnico Nacional, Mexico City, Mexico;3. Centro de Investigaciones Regionales “Dr. Hideyo Noguchi” Universidad Autonoma de Yucatan, Merida, Yucatan, Mexico;4. Facultad de Medicina, Universidad Nacional Autonoma de Mexico (UNAM), Mexico City, Mexico |
| |
| Abstract: | 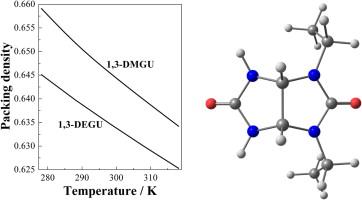
Densities of aqueous solutions of achiral 1,3-dimethylglycoluril (1,3-DMGU) and 1,3-diethylglycoluril (1,3-DEGU) were measured using a hermetically sealed vibrating-tube densimeter, with an uncertainty of 1 · 10−5 g · cm−3, at T = (278.15, 288.15, 298.15, 308.15, and 318.15) K and p = (99.6 ± 0.8) kPa. The solute molality was ranged from (0.06 to 0.39) and from (0.01 to 0.07) mol · kg−1 for the aqueous 1,3-DMGU and 1,3-DEGU, respectively. The standard (at infinite dilution) molar volumes and isobaric expansibilities for the 1,3-dialkyl-N-substituted glycolurils compared in water were calculated and discussed in comparison with the previously derived molar enthalpies and heat capacities of their dissolution (hydration). The temperature-dependent behavior of packing-related hydration effects was described taking into account the structural features of a solute molecule. |
| |
| Keywords: | 1,3-Dimethylglycoluril 1,3-Diethylglycoluril Aqueous solutions Standard molar volumes and expansibilities |
| 本文献已被 ScienceDirect 等数据库收录! |
|

