Epitope Ligand Binding Sites of Blood Group Oligosaccharides in Lectins Revealed by Pressure-Assisted Proteolytic Excision Affinity Mass Spectrometry |
| |
| Authors: | Yannick Baschung Loredana Lupu Adrian Moise Michael Glocker Stephan Rawer Alexander Lazarev Michael Przybylski |
| |
| Institution: | 1.Steinbeis Centre for Biopolymer Analysis and Biomedical Mass Spectrometry,Rüsselsheim am Main,Germany;2.Department of Immunology,University of Rostock,Rostock,Germany;3.Department of Chemistry and Steinbeis Center for Biopolymer Analysis and Biomedical Mass Spectrometry,University of Konstanz,Konstanz,Germany;4.Thermofisher Scientific,Darmstadt,Germany;5.Pressure BioSciences, Inc.,South Easton,USA |
| |
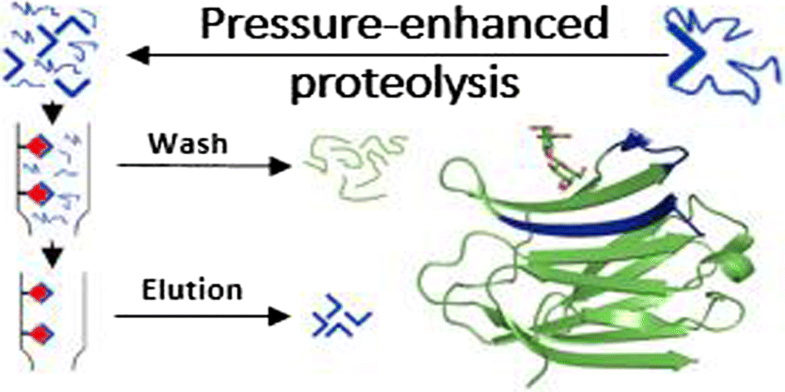
| Abstract: | Affinity mass spectrometry using selective proteolytic excision and extraction combined with MALDI and ESI mass spectrometry has been applied to the identification of epitope binding sites of lactose, GalNac, and blood group oligosaccharides in two blood group-specific lectins, human galectin-3 and glycine max lectin. The epitope peptides identified comprise all essential amino acids involved in carbohydrate recognition, in complete agreement with available X-ray structures. Tryptic and chymotryptic digestion of lectins for proteolytic extraction/excision-MS was substantially improved by pressure-enhanced digestion using an automated Barocycler procedure (40 kpsi). Both previously established immobilization on affinity microcolumns using divinyl sulfone and coupling of a specific peptide glycoprobe to the gold surface of a biosensor chip were successfully employed for proteolytic excision and extraction of carbohydrate epitopes and affinity measurements. The identified epitope peptides could be differentiated according to the carbohydrate employed, thus demonstrating the specificity of the mass spectrometric approach. The specificities of the epitope ligands for individual carbohydrates were further ascertained by affinity studies using synthetic peptide ligands with immobilized carbohydrates. Binding affinities of the synthetic ligand peptides to lactose, in comparison to the intact full-length lectins, were determined by surface acoustic wave (SAW) biosensor analysis and provided micromolar KD values for the intact lectins, in agreement with results of previous ITC and SPR studies. Binding affinities of the epitope peptides were approximately two orders of magnitude lower, consistent with their smaller size and assembled arrangement in the carbohydrate recognition domains. |
| |
| Keywords: | |
| 本文献已被 SpringerLink 等数据库收录! |
|