Transferring Ions from Solution to the Gas Phase: The Two Basic Principles |
| |
| Authors: | Sebastiaan F. Teunissen Marcos N. Eberlin |
| |
| Affiliation: | 1.ThoMSon Mass Spectrometry Laboratory,University of Campinas–UNICAMP,Campinas,Brazil |
| |
| Abstract: | ![]()
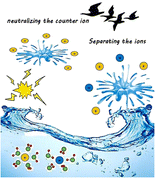
The efficient formation of gaseous ions is the crucial step in all successful mass spectrometric experiments. The invention of electrospray ionization (ESI) has strongly facilitated this step by transferring preformed ions directly from solution to the gas phase – thereby circumventing the need to first convert analytes to the gas phase and then ionize them – and therefore ESI has become an extremely useful and widely applied MS technique. The invention of sonic spray ionization (SSI) has also allowed for the transfer of ions from solution into the gas phase, but without the assistance of a voltage or heating. Numerous ionization techniques, using similar principles to those applied in either ESI or SSI, have subsequently been developed. Although experimental conditions used in such techniques vary markedly, herein we argue that they are all based on either one of two basic principles by which ions can be transferred from solution to the gas phase, that is: via (1) neutralizing the counter ion, or (2) separating the ions. We have selected 35 such techniques and categorized them accordingly. This article thereby aims to establish the basic principles by which gaseous ions can be obtained from solvated ions. We further propose that any new ionization technique used to transfer solvated ions to the gas phase will similarly fall into one of these two mechanistic categories. |
| |
| Keywords: | |
| 本文献已被 SpringerLink 等数据库收录! |
|