|
|||||
|
|
Thermodynamic properties of 5(nitrophenyl) furan-2-carbaldehyde isomers |
|
| Authors: | |
| Institution: | 1.National University “LvivPolytechnic”,Lviv,Ukraine;2.Ivan Franko National University of Lviv,Lviv,Ukraine;3.Charles University in Prague,Prague 1,Czech Republic;4.Lviv,Ukraine |
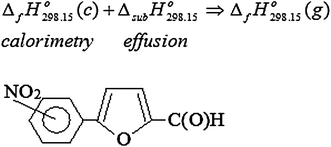
| Abstract: | BackgroundThe aim of the current work was to determine thermo dynamical properties of 5(2-nitro phenyl)-furan-2-carbaldehyde, 5(3-nitro phenyl)-furan-2-carbaldehyde and 5(4-nitro phenyl)-furan-2-carbaldehyde.ResultsThe temperature dependence of saturated vapor pressure of 5(2-nitro phenyl)-furan-2-carbaldehyde, 5(3-nitro phenyl)-furan-2-carbaldehyde and 5(4-nitro phenyl)-furan-2-carbaldehyde was determined by Knudsen’s effusion method. The results are presented by the Clapeyron–Clausius equation in linear form, and via this form, the standard enthalpies, entropies and Gibbs energies of sublimation and evaporation of compounds were calculated at 298.15 K. The standard molar formation enthalpies of compounds in crystalline state at 298.15 K were determined indirectly by the corresponding standard molar combustion enthalpy, obtained using bomb calorimetry combustion.ConclusionsDetermination of the thermodynamic properties for these compounds may contribute to solving practical problems pertaining optimization processes of their synthesis, purification and application and it will also provide a more thorough insight regarding the theoretical knowledge of their nature. Graphical abstract: Generalized structural formula of investigated compounds and their formation enthalpy determination scheme in the gaseous state |
| Keywords: | |
| 本文献已被 SpringerLink 等数据库收录! | |