The effects of power ultrasound (24 kHz) on the electrochemical reduction of CO2 on polycrystalline copper electrodes |
| |
| Affiliation: | 1. Hydrogen Energy and Sonochemistry Research Group, Department of Energy and Process Engineering, Norwegian University of Science and Technology (NTNU), Trondheim, Norway;2. Microelectronics-Photonics Program, University of Arkansas, Fayetteville, AR, USA;3. Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville AR, USA;4. UTINAM UMR 6213 CNRS, Université Bourgogne Franche-Comté, Besançon, France |
| |
| Abstract: | 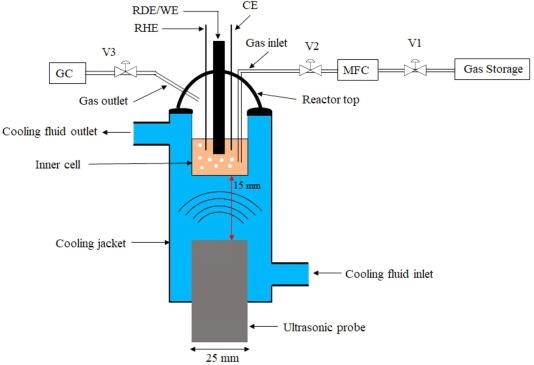
The electrochemical CO2 reduction reaction (CO2RR) on polycrystalline copper (Cu) electrode was performed in a CO2-saturated 0.10 M Na2CO3 aqueous solution at 278 K in the absence and presence of low-frequency high-power ultrasound (f = 24 kHz, PT ~ 1.23 kW/dm3) in a specially and well-characterized sonoelectrochemical reactor. It was found that in the presence of ultrasound, the cathodic current (Ic) for CO2 reduction increased significantly when compared to that in the absence of ultrasound (silent conditions). It was observed that ultrasound increased the faradaic efficiency of carbon monoxide (CO), methane (CH4) and ethylene (C2H4) formation and decreased the faradaic efficiency of molecular hydrogen (H2). Under ultrasonication, a ca. 40% increase in faradaic efficiency was obtained for methane formation through the CO2RR. In addition, and interestingly, water-soluble CO2 reduction products such as formic acid and ethanol were found under ultrasonic conditions whereas under silent conditions, these expected electrochemical CO2RR products were absent. It was also found that power ultrasound increases the formation of smaller hydrocarbons through the CO2RR and may initiate new chemical reaction pathways through the sonolytic di-hydrogen splitting yielding other products, and simultaneously reducing the overall molecular hydrogen gas formation. |
| |
| Keywords: | Sonoelectrochemistry hydrogen evolution reaction (HER) Methane Formic acid Ethanol |
| 本文献已被 ScienceDirect 等数据库收录! |
|

