High pressure effects on benzoic acid dimers: Vibrational spectroscopy |
| |
| Affiliation: | 1. University of Genova, Department of Chemistry and Industrial Chemistry, Via Dodecaneso 31, 16146 Genova, Italy;2. Netherlands Organization for Scientific Research (NWO), DUBBLE CRG, European Synchrotron Radiation Facility, BP 220, F-38043 Grenoble Cedex, France;3. FAMU-FSU College of Engineering, Department of Chemical and Biomedical Engineering, 2525 Pottsdamer St., Tallahassee, FL 32310-6046, USA;1. Crystal Growth and Ceramic Materials Group, São Carlos Institute of Physics, University of São Paulo, CP 13566-590, São Carlos SP, Brazil;2. Department of Materials Engineering, Federal University of São Carlos, CP 13565-905, São Carlos SP, Brazil;1. Poznań University of Technology, Institute of Chemical Technology and Engineering, Pl. M Skłodowskiej-Curie 2, 60-965 Poznań, Poland;2. Silesian University of Technology, Department of Organic Chemical Technology and Petrochemistry, Krzywoustego 4, 44-100 Gliwice, Poland;1. Department of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, IN 46556, United States;2. Laboratory of Computational Chemistry and Drug Design, School of Chemical Biology and Biotechnology, Peking University, Shenzhen Graduate School, Shenzhen 518055, China;1. IFP Énergies Nouvelles-Établissement de Lyon, Catalysis and Separation Division, Rond-point de l‘échangeur de Solaize, BP 3, 69360 Solaize, France;2. IFP Énergies Nouvelles-Établissement de Lyon, Physics and analysis Division, Rond-point de l‘échangeur de Solaize, BP 3, 69360 Solaize, France |
| |
| Abstract: | 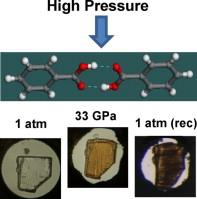
To understand pressure effects on dimer structure stability, Raman and FTIR spectroscopies were used to examine changes in H-bonded dimers of benzoic acid (BA). Experiments were performed on single crystals compressed to 33 GPa in a diamond anvil cell (DAC). Several changes in Raman spectra were observed in the range 6–8 GPa indicating modification in the dimer structure suggesting the lowering of molecular symmetry. Pressure increase above 15 GPa induced strong luminescence and a gradual change of the crystal color from white to yellow/brownish. FTIR measurements on the sample released from 33 GPa indicated formation of a new compound. It is proposed that molecules of this compound are composed of the hydroxyl group associated with alcohol, carbonyl group associated with ketone, and the sp3 hydrocarbon groups. This study demonstrates that sufficient high pressure compression and subsequent decompression can lead to significant changes in the H-bonded dimer structure, including the breaking of bonds and formation of new chemical compound. |
| |
| Keywords: | High pressure Optical spectroscopy Benzoic acid Hydrogen bonding |
| 本文献已被 ScienceDirect 等数据库收录! |
|

